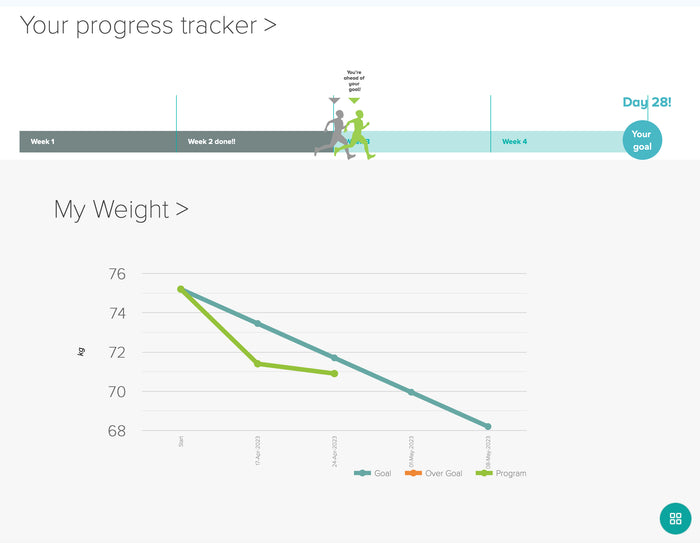
The hugely popular weight loss agent semaglutide (approved as Wegovy for weight loss; Ozempic for type 2 diabetes; Novo Nordisk) received a pair of drug safey-related labeling additions from the US Food and Drug Administration (FDA) in late September for the Ozempic formulation.
The FDA added a warning to the drug-interaction section of the Ozempic label that reiterates a warning that is already in place in other label sections, reinforcing the message that the glucagon-like peptide-1 (GLP-1) receptor agonist Ozempic can potentially interact with the action of certain other agents to increase a person's risk for hypoglycemia.
The added text says: "OZEMPIC stimulates insulin release in the presence of elevated blood glucose concentrations. Patients receiving OZEMPIC in combination with an insulin secretagogue (eg, sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia."
This text was already included in both the "Warning and Precautions" and the "Adverse Reactions" sections of the label. The warning also advises, "The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia."